Overview
Accuracy Matters: The Value of Hemodynamic Monitoring
Hemodynamic monitors are useful in aiding the detection, diagnosis and titration of therapy in response to circulatory shock. An emerging application is the use of hemodynamic monitoring as the input for sophisticated predictive analytic algorithms to help anticipate and prevent the transition of patients into shock. Refractory shock that is not responsive to therapy has an in-hospital mortality of up to 66% and as such, is an emergency.1-3
The current guidelines call for the use of bedside echocardiography upon the diagnosis of shock to determine the cause of shock. Hemodynamic monitoring is then recommended to help further determine the cause of shock and help guide therapy upon the failure of the first line of therapy, typically fluids. However, it is well established that 50% of critically ill patients do not respond to fluids and many require both fluids and vasoactive drug therapy for resuscitation.3, 4
The risks of over- and under-resuscitation are well established. Fluids are routinely administered to account for losses during surgery, to prevent dehydration, and to maximize oxygen delivery to tissue. As shown below, however, there is a “sweet spot” (normovolemia), for fluid management. Too little (hypovolemia) or too much (hypervolemia) fluids are both associated with increased rates of complications.
Figure 1

Cannesson M., J Cardiothorac Vasc Anesth. 2010;24(3):487-497.
The clinical standard of administering fluids has been to administer fluids based on pre-operative weight and “overfill” slightly to increase body weight by 3 – 6 kg. However, as deviations from hemodynamic stability increase (caused by more invasive surgery and/or more severe clinical conditions), it becomes more difficult to choose a fixed dose of fluids to administer in order to achieve normovolemia. At this point, advanced monitoring and goal-directed fluid administration to maintain enough intravascular volume is recommended.
As shown in the Figure 2 (the Starling curve) below, fluid challenges are administered in combination with hemodynamic monitoring for stroke volume (which is Cardiac Output divided by Heart Rate) changes. The goal is to reach Point B, where further administration of fluids does not significantly increase stroke volume.
Figure 2

Adapted from Grocott, Mythen, and Gan, 2005.
More than 32 independently-conducted studies have shown that optimized fluid and drug management with accurate CO monitoring can lead to significantly improved outcomes including reduced mortality, morbidity, and shorter length of hospital stay.3
Why Hemodynamic Monitoring Accuracy Matters
Some clinicians may argue that the choice of hemodynamic monitor used is unimportant, and that following a fluid optimization protocol is what matters. For low-risk and some medium-risk surgical patients who do not have co-morbidities, this approach will likely yield acceptable results so long as the clinician errs on the side of over-resuscitation. Typically, the risks of under-resuscitation are lack of sufficient oxygen delivery, rapidly leading to organ failure, whereas the risk of over-resuscitation is edema, generally leading to impeded bowel function and excess length of stay.5-7
For high-risk patients in the ICU and in high-risk surgeries, however, the situation is much different. These patients have fewer physiologic reserves with which to tolerate pronounced variations in therapy and/or physiologic stressors. The risk of under-resuscitation in these patients is organ failure due to lack of sufficient oxygen delivery. In patients with elevated cardiovascular risk (such as hypertension or heart failure), fluid overload may trigger heart failure, potentially causing cardiac depression, especially in patients with septic cardiomyopathy.8
Taken together, these findings underscore the importance of implementing resuscitation protocols that are based on patients’ actual hemodynamic status rather than adhering to generic weight-based protocols.
Precise Guidance of Hemodynamic Management
Implementation of protocols based on patients’ hemodynamic status requires precise and accurate data. A key challenge in achieving this critical objective is that many hemodynamic monitors do not perform well with respect to tracking changes in cardiac output in response to fluid and vasoactive drug therapy, as shown below.
Figure 3
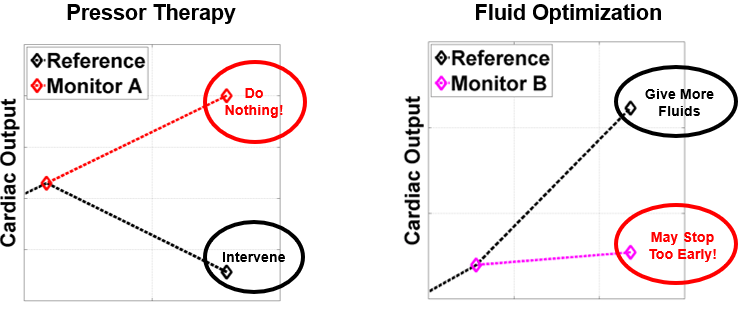
As discussed in the How It Works section, cardiac output monitors that rely on analysis of single beats produce results that are distorted by wave reflection and do not provide accurate results during changes in vasomotor tone 9-11. When titrating pressor therapy, some pressors can increase cardiac output, others may have no effect, and still others may cause decreases in cardiac output due to increased systemic vascular resistance. In these instances, an inaccurate cardiac output, as shown in the left of Figure 3, can lead the clinician to believe that the patient is in an acceptable hemodynamic state, when in fact the patient may require an intervention such as additional fluid support.
Other algorithms are also designed to under-respond to changes in cardiac output. Since many patients are stable for 80 to 90% of the time, a monitor that does not react strongly to changes in cardiac output will have a greater likelihood of producing a plausibly “accurate” result. In the case of fluid optimization, shown on the right of Figure 3, this type of algorithmic approach can cause a monitor to under-react to changes in cardiac output. As shown in the figure, if the monitor reports a small change (under-reacts) in response to a fluid bolus, the clinician may stop administering fluids, incorrectly assuming that the patient is on the flat portion of the Starling curve (Figure 2), whereas in reality, the patient may still require more fluids.
Yet another consideration is accuracy in low cardiac output states. In patients with low cardiac output, oxygen delivery may or may not be sufficient. Accurate estimates of cardiac output may make the difference between how aggressive or conservative a clinician will be in responding to the patient condition, such as choosing to administer or change the dose of an inotrope. While these decisions are usually made in conjunction with other patient data from physiologic monitors, laboratory results and clinical evaluation, it is clear that accurate hemodynamic information is important to guide the diagnosis and therapy for high-risk patients.
- Jenkins CR, Gomersall CD, Leung P, and Joynt GM. Outcome of patients receiving high dose vasopressor therapy: a retrospective cohort study. Anaesth. Intensive Care. 2009; 37(2) 2: 286–289.
- Benbenishty J, Weissman C, Sprung CL, Brodsky-Israeli M, and Weiss Y. Characteristics of patients receiving vasopressors. Hear. Lung J. Acute Crit. Care. 2011;40(3):247–252.
- Jentzer JC, Vallabhajosyula S, Khanna AK, Chawla LS, Busse LW, and Kashani KB. Management of Refractory Vasodilatory Shock. Chest. 2018;154(2):416-426.
- De Backer D. Detailing the cardiovascular profile in shock patients. Critical Care, 2017, 21 (suppl 3):311.
- Hamilton MA, Ceconni M and Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112:1392-1402.
- Grocott MP, Mythen MG and Gan TJ. Perioperative Fluid Management and Clinical Outcomes in Adults. Anesth Analg. 2005;100:1093–106.
- Navarro LHC, Bloomstone JA, Auler JOC, et al. Perioperative fluid therapy: a statement from the International Fluid Optimization Group. Perioper Med. 2015;4(1) published online.
- Richard C, Warszawski J, Anguel N, et al. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. JAMA, 290:2713-2720, 2003.
- Monnet, X. et al. Comparison of pulse contour analysis by Pulsioflex and Vigileo to measure and track changes of cardiac output in critically ill patients. Br. J. Anaesth. 114, 235–243 (2015).
- Meng, L. et al. The impact of phenylephrine, ephedrine, and increased preload on third-generation vigileo-flotrac and esophageal doppler cardiac output measurements. Anesth. Analg. 113, 751–757 (2011).
- Suehiro, K. et al. Systemic vascular resistance has an impact on the reliability of the Vigileo-FloTrac system in measuring cardiac output and tracking cardiac output changes. Br. J. Anaesth. 111, 170–177 (2013).